©
2024
Terms of Service | Privacy Policy
Made with passion and
Huly™
Made with passion and Huly™
Huly.io isn't the only app powered by the Huly Platform. We're also developing TraceX, an open source eQMS tailored for regulated industries like healthcare, where compliance is critical. Both Huly and TraceX leverage the same powerful core functionality, complemented by specialized modules to meet unique needs. Starting with this release, our Huly changelog will now include updates for both products. In this release, discover the latest updates and fixes for Huly.io, along with enhancements to TraceX's controlled documentation system, including improved document management and compliance signing workflows. Check it out!
New icon for desktop: We're excited to introduce our new icon for the Huly desktop app, created by designer Mark Bennett. We're working hard behind the scenes to develop more stunning designs to deliver in the next generation of Huly, but we just couldn't wait to share a little preview of what's to come. #7637
Improved file backup options for desktop and web app: With this release, we’ve enhanced Huly’s backup tool for workspace owners and maintainers. On the desktop app (File > Backup) and web app (Settings > Backup), we provide individual files for download contained in automatic snapshots taken every 12 hours. Each snapshot contains a comprehensive set of files representing your workspace data, ensuring consistent and reliable backups. #7703
Text color palettes: This small enhancement makes it easier to format text colors with a palette of muted tones. Text colors now appear beautifully on both dark and light modes. #7698
Improved interface for mobile devices: As part of our ongoing efforts to improve the Huly experience on mobile devices, this PR introduces updates to the navigator and sidebar for smaller screens. Additionally, a bug with copying text on mobile has been fixed, and the mobile settings layout has been improved. #7694
Inline comments for code blocks and diagrams: This PR resolves a bug that prevented inline comments from being added to code blocks and diagrams. With this fix, users will be able to add inline comments anywhere in their documents, issue descriptions, and anywhere else the comment icon is available in the editor toolbar. #7699
Links in text editor: This PR fixes an issue where a link would automatically be applied to plain text even after the original linked text was removed. #7646
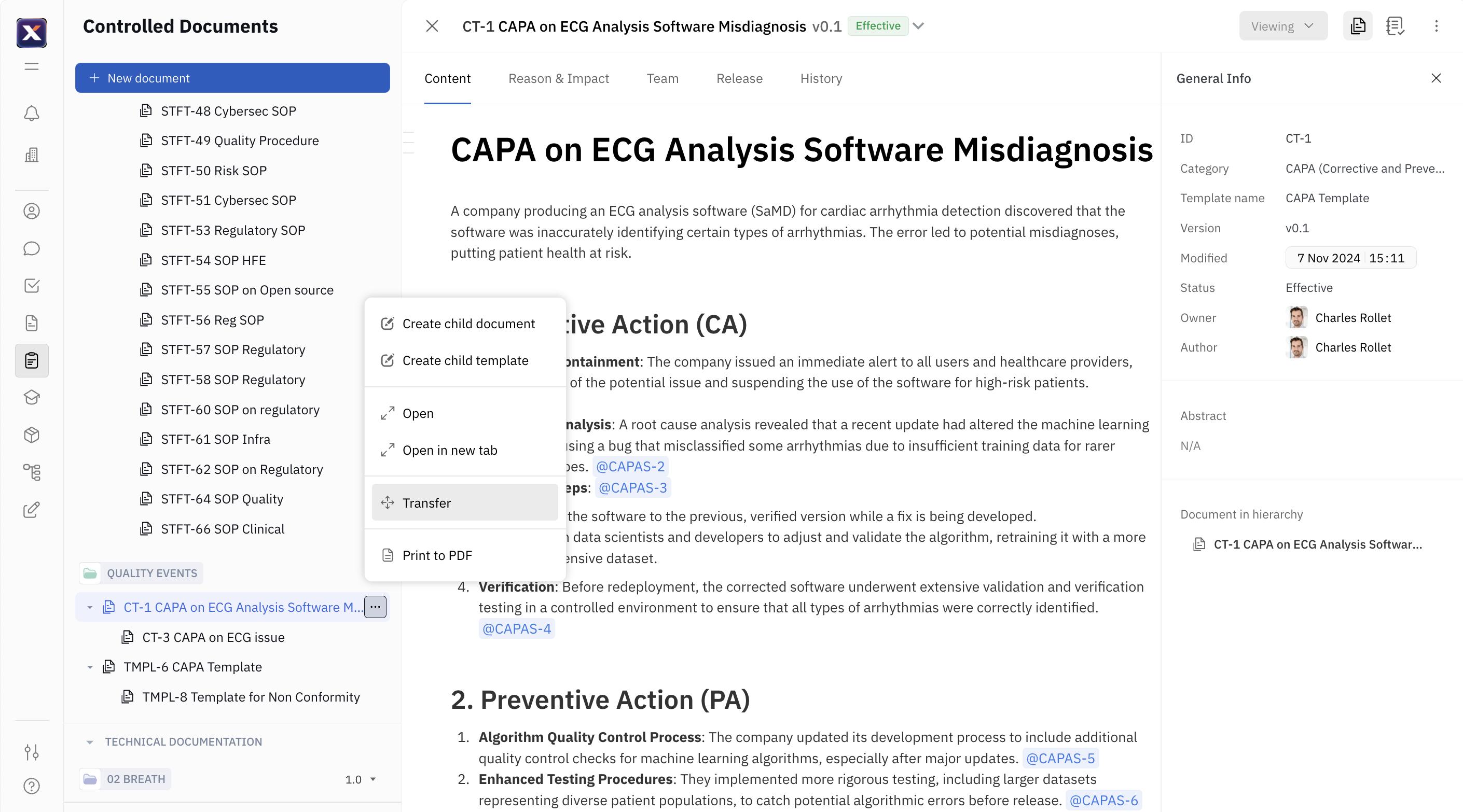
This new eQMS feature enables users to transfer controlled documents between spaces, a critical need for Quality Assurance and Regulatory Affairs (QARAs). It mirrors functionality previously available in uncontrolled spaces while introducing safeguards for controlled environments. Users can move documents only if they meet specific criteria, such as being created in the current product version, and if they have the necessary Role-Based Access Control (RBAC) permissions—specifically, archive rights in the source space and document creation rights in the destination space. The UI supports drag-and-drop functionality within a space, while transfers between spaces utilize a confirmation dialog similar to ownership changes. #7668
This eQMS update introduces workflow adjustments to ensure regulatory compliance during document review and approval processes. Authors are now required to sign documents before sending them for review or approval, with corresponding timestamps included in the document. Additionally, documents that have already been reviewed cannot skip the review step when sent for approval, maintaining the proper order of signatures (Author → Reviewer → Approver). This ensures consistency and helps avoid issues during audits. The exported PDF will reflect the correct signature order based on the review sequence, ensuring proper documentation for regulatory purposes. #7631
Did you know that Huly Docs isn't just for Huly.io? Huly Docs is also home to all of our tutorials on the TraceX eQMS modules. Check out our guides on products, trainings and document review and approval to learn about some of our powerful eQMS capabilities. Learn more at tracex.co.
In this short presentation, TraceX CEO and co-founder Charles Rollet talks about how open source AI-powered software can optimize compliance processes in regulatory industries (e.g., MedTech, BioTech, automotive, and aerospace industries). Built on the Huly Platform, TraceX simplifies the complex regulatory landscape by providing everything your team needs to manage quality processes, technical documentation, and business operations in a single open source solution. TraceX is developing innovative AI-powered tools to keep you compliant, efficient, and audit-ready. You can get in touch with Charles directly through our Slack community on the #eqms channel.